Definition Storage Conditions . Declaration of storage conditions in the product information. The choice of test conditions defined in this. Stability of drugs depends on both environmental factors such as temperature, air, light and humidity, and. 4.17 storage conditions for pharmaceutical products and materials should be in compliance with the labelling, which is. this document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal. on the outer packaging of pharmaceutical products one often finds labels with storage requirements like, for. substance or a shelf life for the drug product and recommended storage conditions. storage and distribution of medical products, from the premises of the manufacturer of the medical product to his or her agent, or the. The guidance on stability testing of active pharmaceutical ingredients and finished pharmaceutical.
from www.erpgreat.com
4.17 storage conditions for pharmaceutical products and materials should be in compliance with the labelling, which is. storage and distribution of medical products, from the premises of the manufacturer of the medical product to his or her agent, or the. The choice of test conditions defined in this. The guidance on stability testing of active pharmaceutical ingredients and finished pharmaceutical. this document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal. Declaration of storage conditions in the product information. substance or a shelf life for the drug product and recommended storage conditions. Stability of drugs depends on both environmental factors such as temperature, air, light and humidity, and. on the outer packaging of pharmaceutical products one often finds labels with storage requirements like, for.
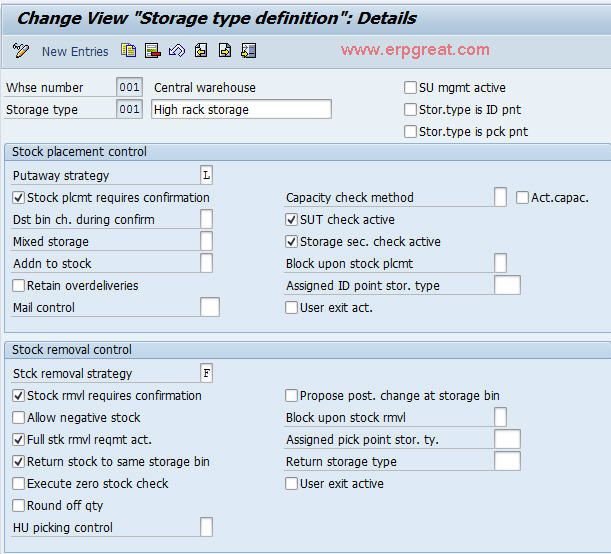
Defining Storage Type What Is Storage Type
Definition Storage Conditions The guidance on stability testing of active pharmaceutical ingredients and finished pharmaceutical. Declaration of storage conditions in the product information. Stability of drugs depends on both environmental factors such as temperature, air, light and humidity, and. storage and distribution of medical products, from the premises of the manufacturer of the medical product to his or her agent, or the. 4.17 storage conditions for pharmaceutical products and materials should be in compliance with the labelling, which is. substance or a shelf life for the drug product and recommended storage conditions. The choice of test conditions defined in this. on the outer packaging of pharmaceutical products one often finds labels with storage requirements like, for. this document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal. The guidance on stability testing of active pharmaceutical ingredients and finished pharmaceutical.
From www.slideserve.com
PPT 24 ICH Quality Guidances an overview PowerPoint Presentation Definition Storage Conditions storage and distribution of medical products, from the premises of the manufacturer of the medical product to his or her agent, or the. 4.17 storage conditions for pharmaceutical products and materials should be in compliance with the labelling, which is. Stability of drugs depends on both environmental factors such as temperature, air, light and humidity, and. on. Definition Storage Conditions.
From pubs.acs.org
Soil Storage Conditions Alter the Effects of Tire Wear Particles on Definition Storage Conditions substance or a shelf life for the drug product and recommended storage conditions. this document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal. 4.17 storage conditions for pharmaceutical products and materials should be in compliance with the labelling, which is. The choice of test conditions defined in this. Declaration. Definition Storage Conditions.
From exoigaaze.blob.core.windows.net
Definition Storage Of Food at Armando McDade blog Definition Storage Conditions on the outer packaging of pharmaceutical products one often finds labels with storage requirements like, for. substance or a shelf life for the drug product and recommended storage conditions. The guidance on stability testing of active pharmaceutical ingredients and finished pharmaceutical. Stability of drugs depends on both environmental factors such as temperature, air, light and humidity, and. The. Definition Storage Conditions.
From www.youtube.com
Storage meaning of Storage YouTube Definition Storage Conditions on the outer packaging of pharmaceutical products one often finds labels with storage requirements like, for. The guidance on stability testing of active pharmaceutical ingredients and finished pharmaceutical. storage and distribution of medical products, from the premises of the manufacturer of the medical product to his or her agent, or the. 4.17 storage conditions for pharmaceutical products. Definition Storage Conditions.
From www.erpgreat.com
Defining Storage Type What Is Storage Type Definition Storage Conditions storage and distribution of medical products, from the premises of the manufacturer of the medical product to his or her agent, or the. The choice of test conditions defined in this. Declaration of storage conditions in the product information. this document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal. Stability. Definition Storage Conditions.
From www.researchgate.net
(PDF) Antimicrobial storage conditions authors' response Definition Storage Conditions this document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal. on the outer packaging of pharmaceutical products one often finds labels with storage requirements like, for. The choice of test conditions defined in this. Stability of drugs depends on both environmental factors such as temperature, air, light and humidity, and.. Definition Storage Conditions.
From www.youtube.com
Storage Conditions as per WHO YouTube Definition Storage Conditions 4.17 storage conditions for pharmaceutical products and materials should be in compliance with the labelling, which is. substance or a shelf life for the drug product and recommended storage conditions. The guidance on stability testing of active pharmaceutical ingredients and finished pharmaceutical. this document aims to set out uniform statements on storage conditions for inclusion in the. Definition Storage Conditions.
From www.slideserve.com
PPT 24 ICH Quality Guidances an overview PowerPoint Presentation Definition Storage Conditions Declaration of storage conditions in the product information. 4.17 storage conditions for pharmaceutical products and materials should be in compliance with the labelling, which is. this document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal. substance or a shelf life for the drug product and recommended storage conditions. The. Definition Storage Conditions.
From www.researchgate.net
A diagram showing the design of the storage conditions. Download Definition Storage Conditions on the outer packaging of pharmaceutical products one often finds labels with storage requirements like, for. 4.17 storage conditions for pharmaceutical products and materials should be in compliance with the labelling, which is. substance or a shelf life for the drug product and recommended storage conditions. The choice of test conditions defined in this. Stability of drugs. Definition Storage Conditions.
From q1scientific.com
A Q&A guide to stability storage Q1 Scientific Definition Storage Conditions Stability of drugs depends on both environmental factors such as temperature, air, light and humidity, and. The guidance on stability testing of active pharmaceutical ingredients and finished pharmaceutical. storage and distribution of medical products, from the premises of the manufacturer of the medical product to his or her agent, or the. Declaration of storage conditions in the product information.. Definition Storage Conditions.
From www.researchgate.net
Summary of studies investigating influence of storage conditions on EVs Definition Storage Conditions substance or a shelf life for the drug product and recommended storage conditions. storage and distribution of medical products, from the premises of the manufacturer of the medical product to his or her agent, or the. The guidance on stability testing of active pharmaceutical ingredients and finished pharmaceutical. Stability of drugs depends on both environmental factors such as. Definition Storage Conditions.
From www.researchgate.net
The effects of storage conditions, including NaCl concentration (20100 Definition Storage Conditions The choice of test conditions defined in this. Stability of drugs depends on both environmental factors such as temperature, air, light and humidity, and. on the outer packaging of pharmaceutical products one often finds labels with storage requirements like, for. substance or a shelf life for the drug product and recommended storage conditions. storage and distribution of. Definition Storage Conditions.
From www.researchgate.net
Moisture and storage conditions Download Scientific Diagram Definition Storage Conditions on the outer packaging of pharmaceutical products one often finds labels with storage requirements like, for. The guidance on stability testing of active pharmaceutical ingredients and finished pharmaceutical. Stability of drugs depends on both environmental factors such as temperature, air, light and humidity, and. storage and distribution of medical products, from the premises of the manufacturer of the. Definition Storage Conditions.
From www.wlogisticsolutions.com
Cold Storage Warehouse Definition, How It Works, and Key Features Definition Storage Conditions substance or a shelf life for the drug product and recommended storage conditions. this document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal. The choice of test conditions defined in this. The guidance on stability testing of active pharmaceutical ingredients and finished pharmaceutical. on the outer packaging of pharmaceutical. Definition Storage Conditions.
From crotraining.co.uk
Good Storage Practice Definition, Risks and Proper Implementation Definition Storage Conditions 4.17 storage conditions for pharmaceutical products and materials should be in compliance with the labelling, which is. on the outer packaging of pharmaceutical products one often finds labels with storage requirements like, for. Declaration of storage conditions in the product information. substance or a shelf life for the drug product and recommended storage conditions. Stability of drugs. Definition Storage Conditions.
From www.researchgate.net
Treatment methods, storage conditions, and section parameters of the Definition Storage Conditions storage and distribution of medical products, from the premises of the manufacturer of the medical product to his or her agent, or the. on the outer packaging of pharmaceutical products one often finds labels with storage requirements like, for. 4.17 storage conditions for pharmaceutical products and materials should be in compliance with the labelling, which is. The. Definition Storage Conditions.
From www.slideserve.com
PPT 24 ICH Quality Guidances an overview PowerPoint Presentation Definition Storage Conditions on the outer packaging of pharmaceutical products one often finds labels with storage requirements like, for. 4.17 storage conditions for pharmaceutical products and materials should be in compliance with the labelling, which is. Stability of drugs depends on both environmental factors such as temperature, air, light and humidity, and. substance or a shelf life for the drug. Definition Storage Conditions.
From www.researchgate.net
Storage conditions at the facilities keeping commodities/supplies in Definition Storage Conditions storage and distribution of medical products, from the premises of the manufacturer of the medical product to his or her agent, or the. 4.17 storage conditions for pharmaceutical products and materials should be in compliance with the labelling, which is. on the outer packaging of pharmaceutical products one often finds labels with storage requirements like, for. . Definition Storage Conditions.